In the world of working scientists, it is very common to conduct experiments or surveys to support your hypothesis. This is the aspect of science that many tend to overlook. Many natural scientists (such as but not limited to: biologist, ecologists, and geologists) call these experiments “Field Work.” This type of work involves very long days that include traveling, working long hours (12+ hour work days), and being able to adapt to equipment failures, uncooperative weather, and many other unplanned events. Also, you must be willing to work hard and keep a positive attitude because field work is not for the weary. While this is not a very educational blog, it is important to understand the complexities of collecting data to support or disprove your hypothesis (both supporting or disproving your ideas are equally important!!!). I just wanted to shine some light on this subject to get a better understanding of what kind of work is involved in the world of scientific research.
Nuclear Power Plant on Hold
A story reported by Lindsey Smith of Michigan Radio on the Palisades Nuclear plant south-west of Grand Rapids was shut down after leaking radioactive water again. This blog will soon be followed up with a short explanation of how radioactive decay works so that we can better understand the risk associated with radioactive materials.
Sources:
Water Quality Constituents
From the blog yesterday, I posted the following water quality report from the Saginaw Waste Water Treatment Plant:
| Test | NPDES Permit Limit | Actual Discharge 2012 |
| Suspended Solids | 30 mg/L | 5.3 mg/L |
| BOD | 30 mg/L | 7.2 mg/L |
| Dissolved Oxygen | 3.0 mg/L | 6.5 mg/L |
| Fecal Coliform | 200/100 ml | 45/100 ml |
| Phosphorus | 0.75 mg/L | 0.39 mg/L |
| pH | 6.5 to 9.0 | 7.1 |
| Ammonia Nitrogen | 5.0 mg/L | 0.4 mg/L |
After noting that each test was passed by the treatment plant, I now want to explain what each of these components are and the importance they hold.
Suspended Solids: Suspended solids are particles in water that cannot be dissolved and are measured as milligrams per Liter. This is primarily a measurement of sediment in the water but can also be irregular particles such as trash or undissolved organic material (scraps of leaves, grass, sticks, etc). This is important because large fluxes of sediment into the water column can suffocate fish, block sunlight from reaching the bottom of the water column and reduce the ability for vegetation to grow.
BOD, Biological Oxygen Demand: BOD is the amount of oxygen required to support aquatic life and is measured as milligrams per Liter. While fish and plants need oxygen to live , it is the algae and molecular lifeforms in water that have the greatest impact on oxygen demand. Large growth events of molecular life forms a spike in BOD and quickly use all the available oxygen in the water and can cause massive fish kills.
Dissolved Oxygen: DO is the counter part to BOD and is measured as milligrams per Liter. DO is the measure of the amount of oxygen available in water. That is also why the test limit of 3 is actually a minimum and not a maximum amount.
Fecal Coliform: This is a test that detects the presence of fecal bacteria and is measured as the number of bacterial colonies per 100 milliliters of water. The main purpose of this test is to determine if bacteria levels are high enough to cause illness to wildlife or impact human health. The most common bacteria that causes illness is E. Coli.
Phosphorus: Phosphorus is a primary nutrient for plants and molecular lifeforms (such as algae) and is measured in milligram per liter of water. The importance to measure phosphorus is to reduce the chance of having algal blooms in the waterways. Algal blooms are sudden and intense growths of algal communities which cause a spike in BOD which can then result in dangerously low oxygen levels (called hypoxia or anoxic water conditions). Some algal blooms can even contain toxins that can kill fish, birds, dogs, and sometimes humans; these blooms are know as Harmful Algal Blooms (HABs).
pH: pH is the measure of hydrogen atoms available for transfer. The “p” in pH means -log (meaning the negative logarithm of Hydrogen ion concentration). liquids with a low pH have a large supply of Hydrogen ions available for transfer and are commonly known as acids. Liquids with a high pH have a low supply of Hydrogen ions and are commonly known as bases. A neutral substance has a pH of 7 on a scale ranging from 1 to 14.
Ammonia Nitrogen: Ammonia Nitrogen in another primary nutrient to plant and algal growth and are measured as milligrams per liter of water. Like Phosphorus, a surplus of Ammonia Nitrogen can cause algal blooms resulting in a spike in BOD resulting in hypoxic conditions and possibly fish kills.
Now we know why these constituents are important to measure as waste water is treated and released back into rivers and lakes.
For more information, here is a link to a water quality page from the EPA that explains these concepts to greater detail, if you are interested: http://water.epa.gov/type/rsl/monitoring/vms50.cfm
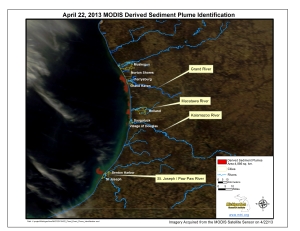
Sediment Plumes Identified in Lake Michigan
To follow through with the graphic I shared a couple of days ago, The Michigan Tech Research Institute (MTRI) has also identified the extent of the sediment plumes in Lake Michigan. The plumes from the Grand, Kalamazoo, St. Joseph, Paw Paw, and Macatawa Rivers covered nearly 5,000 sq. kilometers (That’s Huge)!
Until the MODIS (Aqua) or VIIRS (Suomi NPP) satellite sensors can get another cloud-free look at Lake Michgan, this will be the extent of MTRI’s work on this flood event.
You can learn more about MTRI at http://www.mtri.org and some of the projects I have worked on be visiting http://www.glosaocmapping.org and
http://geodjango.mtri.org/static/sav/
Thanks for reading,
Nate Jessee
Floods Release Sediment in Lake Michigan
Many people in the Great Lakes region have seen a lot of rain lately. As a result many rivers have entered their flood stage. The Grand, Kalamazoo, St. Joseph, and Paw Paw Rivers have released large plumes of sediment into Lake Michigan as a result. Check out the graphic generated by the Michigan Tech Research Institute (MTRI) 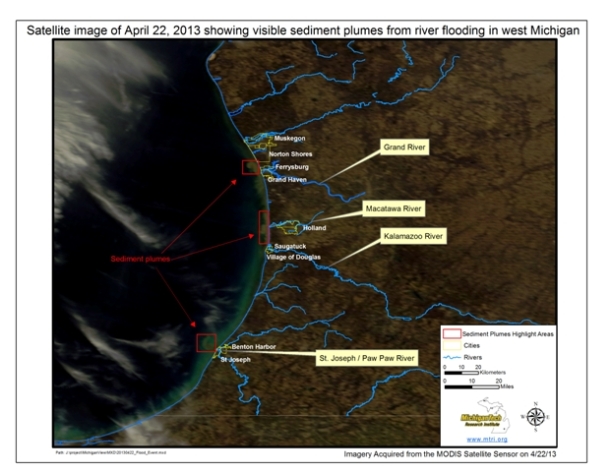 More information about MTRI can be found at http://www.mtri.org and some of the remote sensing work they do can be found here http://www.glosaocmapping.org Lastly, you can follow the Great Lakes Remote Sensing work at MTRI through their twitter feed. MTRI Great Lakes@MTRI_GLOS_AOCs
More information about MTRI can be found at http://www.mtri.org and some of the remote sensing work they do can be found here http://www.glosaocmapping.org Lastly, you can follow the Great Lakes Remote Sensing work at MTRI through their twitter feed. MTRI Great Lakes@MTRI_GLOS_AOCs
Flooding
With all the rain we have seen in Michigan lately, many rivers have flooded; but did you know that is what rivers are supposed to do? As I get my thoughts together, think about where all the water goes during a heavy storm event.
Water Use and You!
So, now that we know that only 1% of all the water on Earth is accessible and usable for all living things, how do you use your water? According to the Institute of Medicine (IoM) an adult male needs to consume about 11 cups and the average adult female needs to consume about 9 cups of water per day; so lets take the average and say an adult needs to consume 10 cups of water per day. So, assuming a 30 day month, an average adult will need to consume 300 cups (or 2400 fl. oz.) per month. I don’t believe I drink this much water, but let’s pretend I do.
OK then, I consume 300 cups (where 1 cup is 8 fl. oz.) per month. I live with my wife and 2 cats. So, my household monthly water needs for my wife and I are 600 cups plus the water our cats drink; and for this exercise lets assume the cat’s consumption is negligible. So, per month my wife and I need 600 cups of water. I just got my water bill yesterday, and according to my water bill, we used 300 cubic feet of water.
So, 300 cubic feet of water……. How much water is that? Put on your thinking caps, cause here come some conversions.
1 cubic foot of water (imagine a container where each side is foot long, and now fill it with water)
How many gallons are in a cubic foot? 1 cubic foot = 7.48 gallons
How many cups of water in one gallon? 1 gallon of water = 16 cups of water
Now that we have our conversions, let’s do the math.
Me and my wife need 600 cups of water. 600 cups is equal to 37.5 gallons (600/16). So, me and the better half need 37.5 gallons of water per month to be adequately hydrated.
Our total water usage for this last month was 300 cubic feet, how many gallons is that? 300 cubic feet of water is equal to 2,244 gallons (300 * 7.48).
So, our total water usage for one month is 2,244 gallons and we only drank 37.5 gallons. So, for all the water we used, only 1.7% of this water was used for drinking.
This means that 98.3% of our water goes down the drain. How much water do you use?
Sources:
(IoM, http://www.iom.edu/~/media/Files/Activity%20Files/Nutrition/DRIs/DRI_Electrolytes_Water.pdf)
Where is all this Water?
As we have previously learned, on Earth there are more than 300 million cubic miles of water (current approximation is 332.5 million cubic miles) . The 332.5 million cubic miles of water is equal to 366 quadrillion gallons of water! Now we must consider where all this water is. Let’s look at the basic sources. First the obvious ones: oceans, lakes, rivers, and clouds. There are 2 not so obvious but significant sources of water: Glaciers and groundwater.
So where is all this water? Let’s get the breakdown from the USGS (United States Geological Survey) water education page.
The Oceans contain 96 – 97 % of all the water on Earth.
Lakes, Rivers, Groundwater, Clouds and Glaciers contain the other 3 – 4 %
Of the 3 – 4% of water contained in lakes, rivers, groundwater, clouds and glaciers, about 98% of that water is in the forms of glaciers, permafrost, and unavailable groundwater (some groundwater is available and others are not). This leaves less than 1% of water on Earth for use by all humans, animals and plants.
So, let’s think about water!
Sources:
How Much Water?
Earth, the blue planet. Sure, we found other planets that have, in one way or another, some form of water; however, as we know it, no other ‘rock’ flying around the universe holds the necessary supply of water to support life. With this introduction, I am starting a series of blogs on water. To start, we will explore how much water is on Earth.
According to the United States Geological Survey (USGS, a section of the Department of the Interior, DOI) there are about 332,500,000 cubic miles of water. OK, great!, but how do you try to envision this enormous volume of water. Try this, in your mind, image a fence that is 1 mile long (feel free to measure a major freeway on Google Earth if you want a more accurate mental picture). OK, got it. Now, using 3 more sections of fence, draw a square. Now, instead of just a fence, turn it into a building that each side is one mile long, but also is one mile tall. This structure we have just imagined in 1 cubic mile. So if this structure were a bucket, it would hold 1 cubic mile of water. Now, imagine 300 million of these buckets. That is the approximate amount of water on Earth. This water makes up our oceans, lakes, rivers, clouds, plants, underground, our bodies, and everything else. Next, we will look at where all this water is.
Source:
USGS Water information page: http://ga.water.usgs.gov/edu/earthhowmuch.html
Water Water Everywhere
As I am getting my thoughts together, I wonder what your thoughts are. What do you know about water? If you have the time, post a comment on something you know about water. besides freezing at 32/0 degrees and boiling at 212/100 degrees, what else do you know about water? Make a list of uses for water. How much water do you use? Let’s think about water today.